Ferrocalm: First probiotic on the market to support gut health during stress or an IBS flare up
Regulatory exemptions (GRAS in USA, QPS in Europe) allowed us to launch a food supplement product containing Streptococcus thermophilus
FX856 in 2022: Ferrocalm
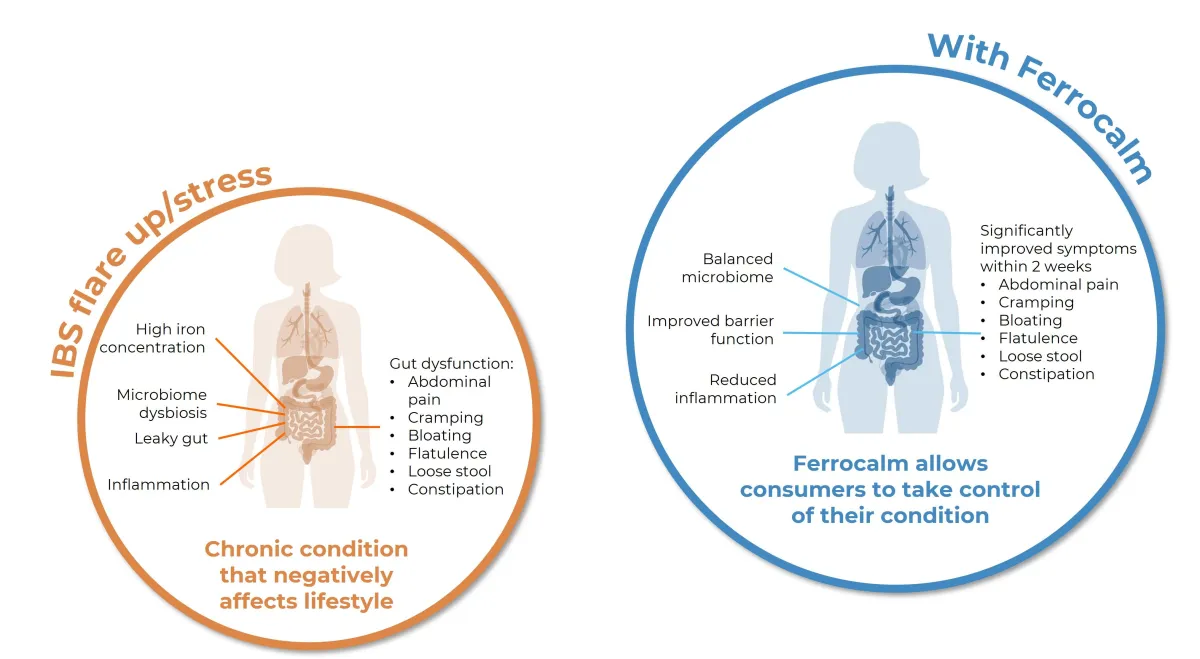
In our trials, 76% of consumers reported an improvement to their symptoms within eight weeks and 88% would take it again
Allowing consumers to manage their gut and claim back their life
Regulatory exemptions (GRAS in USA, QPS in Europe) allowed us to launch a food supplement product containing Streptococcus thermophilus
FX856 in 2022: Ferrocalm
In our trials, 76% of consumers reported an improvement to their symptoms within eight weeks and 88% would take it again
Allowing consumers to manage their gut and claim back their life
How it works
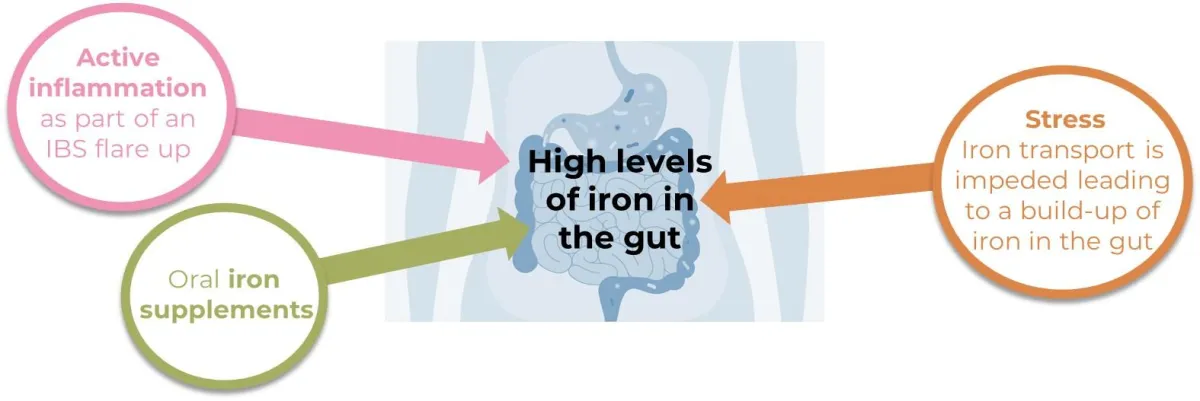
The majority of bacteria present in the gut can use iron as a growth factor and they rapidly multiply under high iron conditions
Conventional probiotics cannot use iron as a growth factor, can't compete for nutrients, and are therefore crowded out
Our unique strain of bacteria, Streptococcus thermophilus FX856, can use iron as a growth factor and can therefore survive and thrive in a high iron environment
FX856 can survive and thrive in a high iron gut
and support gut health
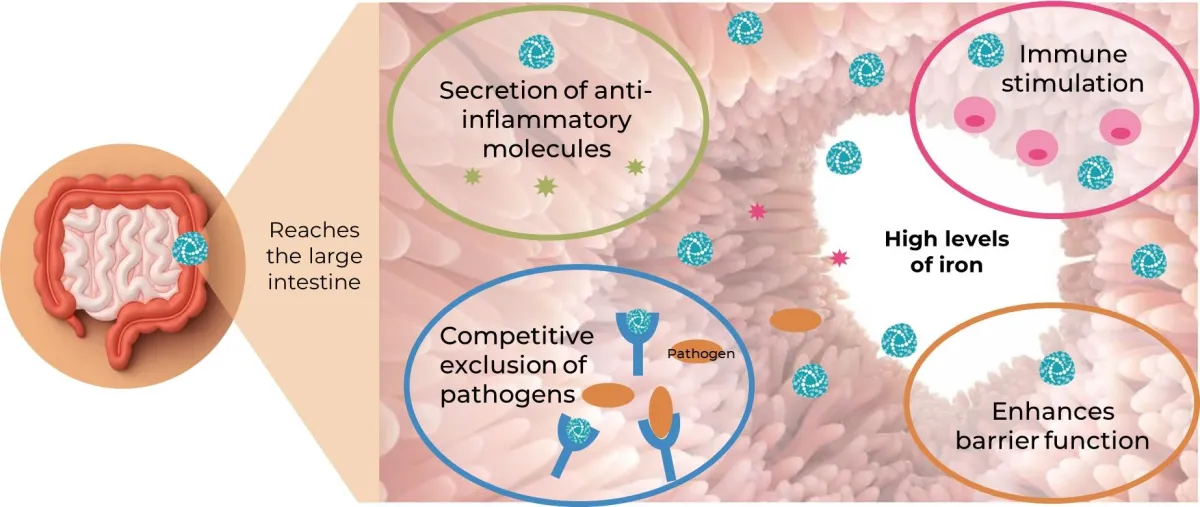
Ferrocalm restores balance to support gut function
FERRYX LTD
Future Space,
UWE North Gate,
Filton Road,
Bristol,
BS34 8RB.


